Crispr, the genetic revolution we can eat right now
Gene editing is a reality in livestock and agriculture, whether it's fish without bones, non-rotting fruit, or gluten-free wheat.
Its application to the treatment of human illnesses is still in its early stages.
Bacteria developed a highly effective virus defense 3,000 million years ago: whenever a virus enters their body, they store a portion of their genetic material in a kind of 'library,' from which they select the appropriate 'tome' each time. Whenever a virus that is similar to this one tries to infect them, they will be quarantined. They use this information to search your DNA strands, removing the harmful sequence embedded within and halting the disease in its tracks. A simple method that science only recently discovered. Francis Mojica, a Spaniard, discovered it was a type of bacteriophage "natural self-vaccine" in the summer of 2003. 'Clustered Regularly Interspaced Short Palindromic Repeats,' or CRISPR, was his name for it.
Years later, in 2012, researchers Emmanuelle Charpentier and Jennifer Doudna published a paper in'Science' describing how to transfer this mechanism to other living things, earning them the Nobel Prize in Chemistry in 2020. This was the beginning of a scientific revolution that is still unfolding. Eat, too.
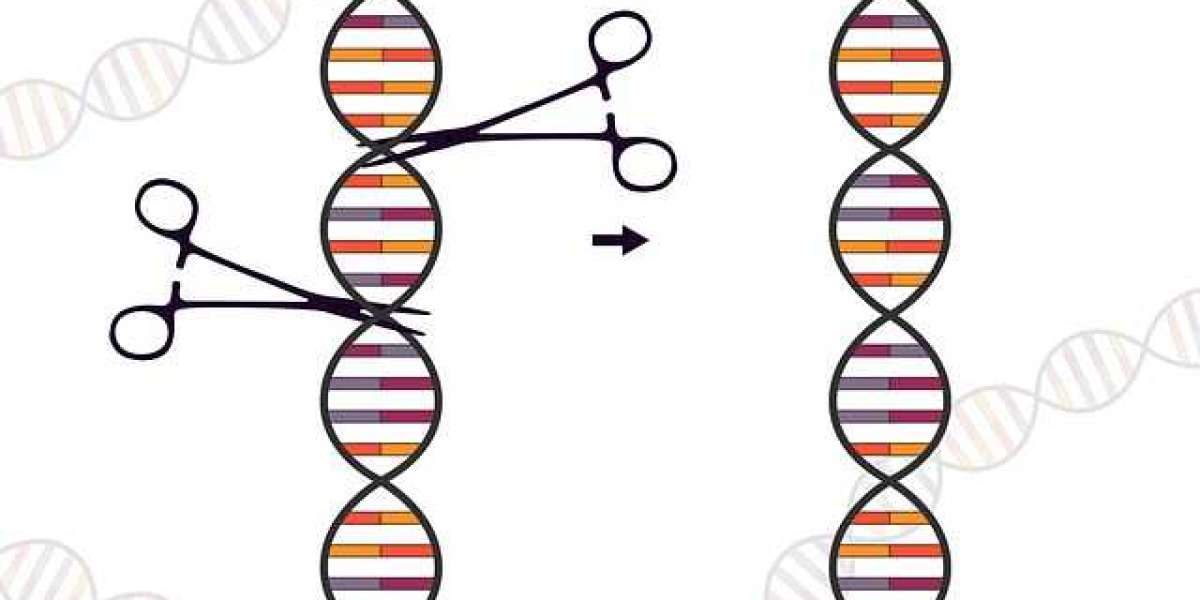
CRISPR technology, also known as 'genetic cut-paste,' is a type of'scissor' made up of guides and a protein (the most well-known is Cas9, but others like Cas12 or Cas10) that can be programmed to look for, join, and cut a specific sequence of DNA. Something that allows almost any cell to be edited and created in the lab from malaria-carrying mosquitoes without the 'genetic impulses' that force them to bite people, passing through disease modeling in the lab to study diseases like never before, or the (yet) promise of therapies that eliminate disease-causing genes.
What is a 'cutter' in genetics?
CRISPR is a gene editing system that works in a similar way to the human genome.
This method uses guides and a protein (Cas9, though other proteins with different properties, such as Cas 10 or Cas 12, have already been used) to cut specific sections of DNA. The gene can then be inactivated or DNA templates inserted, allowing its "letters" to be edited at will.
What applications do you have?
It can be used to change the sequence of DNA in almost any situation. As a result, it is proving to be very useful in basic research in terms of generating disease models that were previously difficult to study, as well as studying new targets and drugs. It also enables the creation of new plants in which an existing gene is modified rather than a new one being introduced - as is the case with transgenics.
What constraints do you have?
The technology is still being fine-tuned: sometimes larger fragments are cut than desired, or DNA rearranges itself in unexpected ways, causing unexpected changes. This is the most significant impediment to its human application.
Is there anything ethical about it?
Yes. CRISPR has sparked a slew of ethical concerns since its inception, particularly since the case of the Chinese scientist who altered human embryos from which three girls were born. The method allows for the creation of 'humans à la carte,' with enhanced learning and memory capabilities. As a result, many researchers request a comprehensive discussion among all scientists.
"I would have said all of this was impossible a few years ago," says Lluis Montoliu, a researcher at the CSIC's National Center for Biotechnology (CNB-CSIC) and one of Spain's leading technology experts. He explains that his team discovered CRISPR "by serendipity," a fortunate find that occurred because it was in the right place at the right time: an Italian student on his team was in Switzerland for a temporary stay at the same time as this new tool arrived. "After learning everything he could, he returned." Our lives were changed by him». They were able to give a voice to experiments that had been going on for two decades with other, much more complex and expensive methods, with little success. "At first, we didn't believe it because it worked so well," he says.
The researchers are using CRISPR to investigate albinism, a disease that we are familiar with because of the lack of pigmentation in those who suffer from it, but which also manifests other more serious problems such as low visual acuity (the European Union considers them legally blind). Montoliu conducts genetic tests on Spanish patients and their families to determine which gene is affected (there are up to 22 types), and then creates the 'avatar mouse,' which accurately reproduces the disease and allows for in-depth research. "The user's imagination is the limit of this technology," she says.
In fact, clinical trials for cancer, two common blood diseases (sickle cell anemia and beta-thalassemia), and the deadly disease transthyretin amyloidosis are already underway. Hundreds of other studies are awaiting approval for human testing. "There are no bounds to it." Each bacterium has its own CRISPR system, and there are millions of them. And you won't have to look far to find them: in the pot on your balcony, you'll find tens of thousands of candidates for creating a new tool, who knows with what peculiarity».
Threats posed by biohackers
Miguel Angel Moreno Mateos, a molecular biologist, moved to Yale University in 2013 to pursue his second postdoctoral degree. "Come on, take a look at this CRISPR thing," his boss, renowned researcher Antonio Giráldez, told him. "He didn't even know what it was," Moreno Mateos admits to ABC, "and he certainly didn't imagine the revolution of this tool." Using the zebrafish as a model, his team at the Andalusian Center for Developmental Biology studies the early stages of embryonic development. "The ovule, the maternal contribution, is responsible for activating the zygotic genome, which is silent at first." They use CRISPR technology to activate and deactivate genes in order to figure out exactly what causes a new living being to emerge.
A living being that, in the future, thanks to genetic technology, will be possible to create artificially. In fact, Chinese scientists claimed this week that they had used this novel technique to create a mouse pup from the egg of a female who had never had sex or received sperm from a male. It's the first instance of artificial parturition in a mammal, which was thought to be impossible until recently.
"We're transforming science fiction into reality," says Moreno Mateos, who also emphasizes the system's democratization, owing to its ease of use and low cost: a CRISPR kit can be purchased for just 100 euros. However, despite being a true revolution, he emphasizes that it will not be the panacea for all of our problems: "Having a little pill that will fix us will be very difficult," he says. It also comes with its share of dangers. The most egregious incident was that of Chinese researcher He Jiankui, who went over and beyond by using CRISPR to modify multiple human embryos, three of whom were born. He is currently incarcerated, and little is known of the children who were 'engineered' to be HIV-resistant (an objective that, according to the little existing information, was not achieved).
The whole scientific world condemned the experiment at the time for its complete lack of ethics. The geneticist George Church, who claimed his "right to a balanced opinion," was the only one who disagreed. "Before we accuse, let's do the math" (...) We have pigs with hundreds of CRISPR mutations and a mouse strain with 40 CRISPR sites that are always active, but no evidence of harmful consequences," the researcher maintained in an interview with'science.' He is the driving force behind the contentious initiative to'resurrect' mammoths by putting their genes into the DNA of a living elephant. But not with the goal of resurrecting this extinct animal; instead, he wants to send these woolly elephants to Siberia to cut down trees, trample the ground, and create a "vegetable carpet" that will protect the permafrost and prevent thawing.
"To be sure, it's a revolution. And the potential of this technology is huge. "However, we also have a responsibility to educate society and tell it that being a "biohacker" leads nowhere," Moreno Mateos argues.
'Flavor' in CRISPR
Crispr uses in livestock and agriculture, on the other hand, are now a tangible (or, rather, edible) reality. Lambs and cows modified to produce tastier meat, mushrooms that stay longer without turning black, apples that do not decay when they fall to the ground, tomatoes that assist reduce hypertension, and boneless fish, as recently achieved in China by a group of researchers. They did it with crucian carp, a famous fish for its soft meat but difficult to handle due to its small intramuscular bones that can get lodged in the throat.
In Spain, a group directed by Francisco Barro Losada of the CSIC's Institute for Sustainable Agriculture is developing gluten-free wheat. "We inactivate the gene that causes gluten; it is not produced in the plant, and the wheat it produces is safe for celiacs," Barro Losada says to ABC. He clarifies that this wheat is not genetically modified, but rather undergoes a redesign of the plant that will eventually produce the grain. "It's the same process that humanity has been performing for the previous 10,000 years; CRISPR is just a tighter management of it all." It is a tool, not a goal, according to the researcher. "It's like when you used to operate with a saw, and now you use a scalpel."
Although this appears to be an issue on which not all governments agree. While Japan and the United States almost immediately legislate to regulate these crops and their commercialization as new advances emerge, the European Union is wary: this type of food, which uses CRISPR, is classified as genetically modified (in the same category as the controversial transgenic, though in the case of the 'genetic glue,' genes aren't implanted, but only cut), and its cultivation is prohibited. Importation from other countries, however, isn't the case. "We can buy them, but we can't grow them here and manage the supply." "We're squandering a big potential," says Barro Losada, who must conduct his experiments in South American plantations as a result. "It doesn't make sense since it'll be the instrument of the future: It'll let us to design crops that are more heat resistant or require less water, which is critical in the face of climate change." People must overcome their apprehensions about technology, which was designed to assist them.
War on patents
With so much on the line, it's no surprise that a battle for ownership of a patent has erupted. After a long legal battle, the US Patent and Trademark Office (USPTO) recently attributed the invention to Feng Zhang, a neuroscientist at MIT's Brad Institute -which uses CRISPR to treat psychiatric diseases-, leaving out his co-creators, Charpentier and Doudna, who have lost the intellectual property rights to commercialize the technology and the power to decide who will use it, at least in the United States. Regardless of legal disputes, the revolution appears to be unstoppable: CRISPR appears to be here to stay.